Abstract
Introduction: As the RRMM treatment landscape evolves, it is imperative to understand how differences in benefits, risks, and administration influence patients’ preferences for treatment. This study quantified patient preferences to inform healthcare professionals in shared decision-making when choosing between treatments for RRMM.
Methods: Adults with RRMM in the USA, UK, France, Spain, Italy, and Germany completed an online discrete choice experiment (DCE) including 12 experimental and 2 internal validity choice tasks (Feb-Jun 2022). In each task, participants chose between 2 hypothetical treatment profiles with different levels of benefits and risks and modes of administration, guided by evidence on approved treatments for RRMM. Patients also completed sociodemographic, quality of life, and clinical questionnaires. Attributes and levels of interest were informed by a targeted literature review, clinical data on RRMM treatments, qualitative concept elicitation interviews with 19 patients (US, UK, Germany each n=5 and France n=4), clinical expert input, and patient advocacy group feedback. The DCE was refined through 12 patient interviews (n=2 per country) to ensure that attributes and levels were comprehensible. Final DCE attributes included overall response rate (ORR), duration of response (DOR), overall survival (OS), peripheral neuropathy, ocular adverse events, cytokine release syndrome (CRS), severe diarrhea, and mode of administration. Preference data were analyzed using an error-component logit model and relative attribute importance (RAI) scores were calculated (higher values indicate larger effects of an attribute on overall preference).
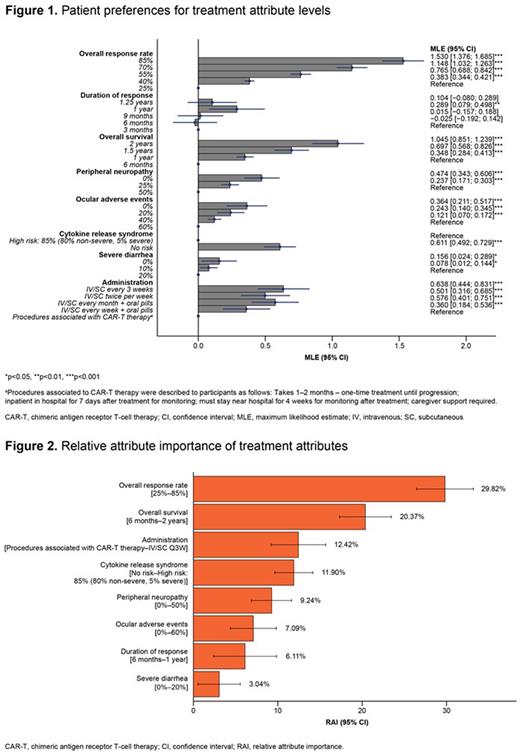
Results: In total, 296 patients completed the DCE (US n=100, UK n=49, France n=39, Spain n=20, Italy n=45, Germany n=43). Mean (SD) age was 64 (8) years, 52% were male, and 84% had a caregiver. Of the US sample 41% were Black/African American and 27% were White. At the time of the survey, patients had received a median of 3 (range 2-8) lines of therapy, were diagnosed on average 5.9 years prior, and most were in either partial (46%) or complete response (31%). When evaluating their quality of life over the past week, 64% of participants reported mild to moderate cancer symptoms (19% severe, 4% very severe), 61% moderate to very severe pain, and 44% severe to very severe fatigue. Figure 1 shows patients’ preferences by treatment attribute level, and Figure 2 describes the overall relative importance of each attribute. Patients significantly preferred treatments with higher ORR and longer OS; increasing ORR from 25% to 85% (RAI: 29.8% [95% CI: 26.5%, 33.2%]) and increasing OS from 6 months to 2 years (RAI: 20.4% [95% CI: 17.3%, 23.4%]) had the greatest influence on treatment preferences. Administration route was also considered an important attribute (RAI: 12.4% [95% CI: 9.2%; 15.7%]). With respect to side effects assessed in this study, patients showed concern for CRS (RAI: 11.9% [95% CI: 9.7%, 14.1%]) and peripheral neuropathy (RAI: 9.2% [95% CI: 6.9%, 11.6%]). Reducing the risk of ocular side effects (from 60% to 0%) was one of the least important attributes to patients (RAI: 7.1% [95% CI: 4.4%, 9.8%]; 1.6x and 1.3x less important than reducing the risk of CRS and peripheral neuropathy, respectively, and 4.2x less important than increasing ORR (from 25% to 85%). Intravenous (IV) or subcutaneous (SC) administration every 3 weeks (Q3W) were preferred over procedures associated with CAR-T therapy (1-2 months including apheresis, bridging therapies, hospitalization, and caregiver support). While ORR was the most important attribute to patients, they would tolerate a 41% reduction in ORR to increase OS from 6 months to 2 years, 25% reduction to switch from procedures associated with CAR-T therapy to IV/SC administration Q3W, and 24% to reduce the risk of CRS from 85% to no risk.
Conclusions: Treatment preferences of patients with RRMM were strongly focused on maximizing ORR and OS, accounting for half the total RAI. Consistent with current medical understanding, patients generally preferred to avoid side effects, but this was less important than increasing efficacy. This study provides insights on patients’ valuation of treatment attributes and should be considered by healthcare professionals to inform shared decision-making in treatment selection.
Funding: GSK (212408)
Disclosures
Popat:Janssen, Takeda, Celgene, and GSK: Honoraria; Takeda: Research Funding; Takeda, AbbVie, GlaxoSmithKline, and Celgene: Consultancy; BMS: Honoraria; Roche: Honoraria; Janssen: Honoraria; GSK: Honoraria, Research Funding; Janssen, Takeda, GSK: Other: Travel expenses from Janssen, Takeda, GSK. Kleinman:Calm Water Therapeutics LLC: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Triphase Accelerator Corp: Consultancy; GSK: Consultancy; Editas Medicine: Consultancy; Ascidian Therapeutics: Consultancy; Prime Medicine: Consultancy; ONL Therapeutics: Consultancy; Helixmith: Consultancy; AGTC: Consultancy; Emergent BioSolutions: Consultancy. Perera:GSK: Current Employment, Current equity holder in publicly-traded company. Gorsh:GSK: Current Employment, Current equity holder in publicly-traded company. Thomas:Evidera: Current Employment; GSK: Research Funding. Mulnick:Evidera: Current Employment; GSK: Research Funding. O'Neill:GSK: Current Employment, Current equity holder in publicly-traded company. Ross:Evidera: Current Employment; PPD and Thermo Fisher Scientific: Current equity holder in publicly-traded company; GSK: Research Funding. Paka:GSK: Current Employment, Current equity holder in publicly-traded company. Hanna:GSK: Current Employment, Current equity holder in publicly-traded company. Molinari:GSK: Current Employment, Current equity holder in publicly-traded company. Naine:GSK: Current Employment, Current equity holder in publicly-traded company. Gelhorn:Evidera: Current Employment; GSK: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal